NQR SPECTROSCOPY
NUCLEAR QUADRUPOLE RESONANCE SPECTROSCOPY
·
Why
we use NQR Spectroscopy?
We
use NQR Spectroscopy to examine Chemical analysis. NQR transition of nuclei can
be detected in the absence of magnetic filed so for this NQR Spectroscopy is
referred as “Zero field NMR”.
 |
| Instrument of NQR Spectroscopy |
Instrument of
NQR Spectroscopy
WHAT IS QUADRUPOLE AND QUADRUPOLE
MOMENT.
·
Any body which is having a four poles
is called a quadrupole body.
·
Quadrupole will decide the shape of a
molecule that whether the Shape of a molecule is in horizontal oval (Q<0)
vertical oval (Q>0).
·
Their spin quantum no. must be (I>1)
·
No. of orientation depend upon the
magnitude of nuclear Quadrupole moment.
·
The NQR Spectroscopy is intermediate
by the interaction of electric filed gradient.
Charge distribution of nucleus will
tell us the shape of molecule.
 |
| the shape of molecule |
QUADRUPOLE MOMENT:
Quadrupole
mechanism consideration of the distribution of nuclear charge shows that nuclei
do not have permanent electric dipole moment but can have electric Quadrupole
moments when their electric charges are not spherically symmetric. This is
usually the Quadrupole moment.
Electric filed gradient:
It
s the parameter which measure the rate of change of electric field in which
atomic nuclei generated by the electric charge distribution nearby an
electronic charge densities on the nucleus of an atom.
·
During spectroscopy it couples the
quadruple moment of nucleus with electric field gradient due to which nucleus
staring to resonate.
·
That’s why we call that it is nuclear
quadrupole resonance spectroscopy in which all the energy levels are generated.
·
Now in nucleus electro spinning is
rise different levels.
·
To study these electrostatic
interaction levels we do NQR spectroscopy.
·
They are directly applied by
nature we don’t provide it any source of UV light, X-ray beam.
·
It is fixed and particular so its is also
called chemical finger print.
MOSSBAUER SPECTROSCOPY
·
It’s light source is gamma
δ-rays so it is also called gamma rays resonance
spectroscopy.
·
In this spectroscopy both ground
state and excited state.
·
It is only occur on solid
objects or a chemicals coordination compounds.
·
The coordination compounds will
be detected on the nuclear energy levels.
·
All compounds are not studied by
Mossbauer spectroscopy. They are specified.
·
Sample will only absorb 14.4 eV.
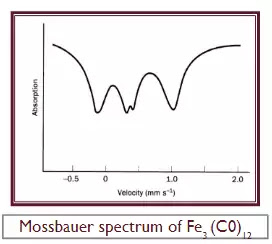
EXAMPLE OF FE3(C0)12.
·
An excellent example of the use of
Mossbauer spectroscopy in structure determination is provided by Fe3(C0)12.
Although the dark green crystals of this compound are easy to prepare dissolve
Fe(C0)5 in
aqueous alkali to give the Fe(CO)4-2
anion and oxidize this with solid Mn02
to give Fe3(C0)12-its structure was
uncertain for over 30 years Although eight structures, all incorrect,
had been proposed for Fe3(C0)12,
it was the ninth, suggested on the basis of its Mossbauer spectrum, which eventually proved to be correct. The most
evident thing is that it corresponds, approximately, to three peaks of equal
intensity.
 |
Mossbauer spectrum of Fe3 (C0)12 |






0 Comments
Thanks You....